Raman analysis and mapping can be used to rapidly probe an oxidized CNTs sample, extracting information on the presence of acidic groups produced by oxidative treatments usually performed to allow both a better dispersion in solution and the further attachment of biomolecules for drug delivery applications. The Raman signal of CNTs can be exploited to identify their presence inside the cellular membrane during in vitro tests, and to study their biodistribution after in vivo tests.
Starting
from the fundamental paper by Ijima in 1991 [Iijima S. Helical microtubules
of graphitic carbon. Nature, 1991, 354, 56-8], carbon nanotubes, both
single and multiwalled ones, have received an ever increasing scientific
and industrial interest due to their exceptional physical and chemical
properties, and have been considered and applied in a growing number
of different fields from electronics to photonics. In particular, their
possible use in the field of biomedicine was recently suggested and
partly demonstrated, turning on a renewed interest and enthusiasm, and
prompting the scientific community to a powerful interdisciplinary approach.
In this context, many methods were developed for attaching molecules
to the inside and outside of the hollow nanotubes, opening the way both
to their use as biosensors and molecular transporters. In particular,
single walled carbon nanotubes (SWCNT) have shown to be able to shuttle
various molecular cargos inside living cells, including proteins, short
peptides, and nucleic acids, having then the ability to target and eventually
destroy individual cells that may be cancerous or infected by a virus.
But, to be used, raw/pristine SWCNTs have to be oxidized, in order to
eliminate toxic metal residues, to enhance their solubility and to increase
the number of carboxylic groups present on their graphitic walls that
can be utilized for further functionalization. Since the abundance of
carboxylic groups, increasing with oxidative treatment time, temperature
and the degree of lattice defectiveness, is then critical for the final
application, it is important to develop a reliable but possibly fast
method to investigate their presence on the tube walls. However, there
are some difficulties associated with monitoring the oxidative reaction.
Although a number of analytical techniques such as IR and XPS have been
applied to address this issue, a routine monitoring tool, without the
need for specialized hardware, is still difficult to find. Some groups
have reported acid-base titration of acidic sites of different CNTs
[M. W. Marshall, S. Popa-Nita and J. G. Shapter, Carbon, 2006, 44, 1137;
H. Hu, P. Bhowmik, B. Zhao, M. A. Hamon, M. E. Itkis and R. C. Haddon,
Chem. Phys. Lett., 2001, 345, 25.]; but these techniques suffer from
several shortcomings, such as the need for a proper dispersion of CNTs
in aqueous solution and the fact that, being multistep sequences, they
do not meet the requirements of a fast routine test.
In collaboration with the Department of Drug Science and Technology
of the University of Turin, we developed a very simple and fast method
[1] for the evaluation of acidic sites on oxidized CNTs based on direct
labeling with the commercially available fluorescent dye thionin acetate
(THA), successfully applied for the quantitative determination of functional
groups on the surface of insoluble polymers [N. Medard et al. Surf.Interface
Anal., 2001,31, 1042-1047; F.Poncin-Epaillard et al. Macromol.Chem.
Phys. 200, 201, 212; V.B Ivanov et al. Surf.Interface Anal., 1996,24,
257]. The proposed procedure is based on the direct one step labelling
of the oxidized CNT by THA: ionic pairs formation occurs on the CNT
between the dye cation (T+) and CNT carboxylate groups (figure 1).
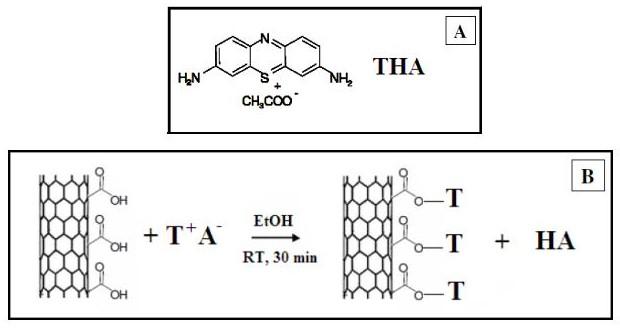
Figure
1. A: Structure of the labelling molecule, thionin acetate
(THA); B: scheme of reaction between THA and oxidized carbon
nanotubes.
Accordingly, after THA solution mixing with oxidized SWCNTs, almost complete decolourization of the supernatant is observed (figure 2). Therefore, the concentration of COOH groups of an oxidized CNT sample can be determined by measuring the fluorescence emission intensity decrease of the initial dye solution, and subtracting the amount due to unspecific interaction (evaluated on the corresponding non-oxidized CNT sample).
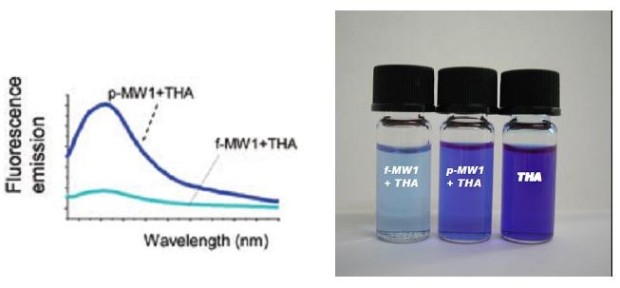
Figure 2. Fluorescence emission spectra (left) on raw (p-MW1+THA) and oxidized (f-MW1+THA) THA treated carbon nanotubes. This figure shows that fluorescence intensity decreases after oxidation due to THA interaction with oxidized nanotubes. On the rigth, the difference in fluorescence emission after mixing THA stock solution and oxidized CNTs (f-MW1) or pristine CNTs (p-MW1).
THA labelling of carbon nanotubes might then allow a semi-quantitative
determination of carboxylic groups induced by the oxidation procedure.
As we
have demonstrated in [2] the presence of the labelling molecules in
the THA treated CNT sample can be also read out by exploiting their
Raman signature [2]. Figure 3 shows the Raman spectrum obtained by using
a Dispersive Raman Spectroscope (DXR Raman spectroscope Thermo Fisher
Scientific, excitation laser 532 nm, laser power 2mW and aperture 50
m pinhole) on a liquid THA sample. It clearly shows a characteristic
peak at about 474 cm-1, whose intensity is related to the number of
molecules present in the analyzed sample.
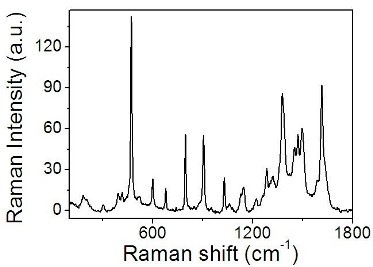
Figure
3. Raman
spectrum obtained on a liquid THA sample.
It is possible to directly use the Raman signal of the THA labelling molecule for the COOH groups determination. We explored the possibility to use our labelling approach to study CNT sample homogeneity and the oxidation treatment efficiency. Figure 4 presents two spectra obtained on different area of a powder sample containing THA treated oxidized SWCNTs, and showing the presence of some of the typical bands ascribed to carbon nanotubes (RBM, D, G, M, G' bands).
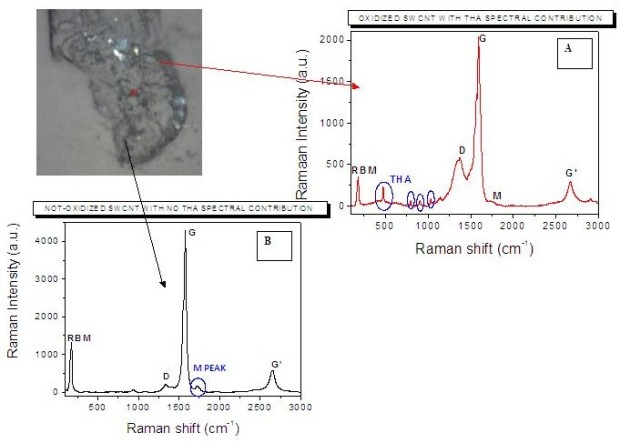
Figure
4.
Up-left: microscopic image of the analysed powder sample containing
THA treated SWCNTs; A: Raman spectrum realized on an area containing
oxidized tubes, showing the typical bands ascribed to carbon nanotubes
(RBM, D, G, M, G' bands) and that assigned to THA molecules; B: Raman
spectrum realized on an area containing pristine tubes, just showing
the typical bands ascribed to carbon nanotubes.
Two spectral features can be chosen to discriminate between oxidized and non-oxidized CNTs. The first one is the intensity of the peak located at 475 cm-1, which is associated to the presence of THA molecules (spectrum A). The second one is the intensity of the M band located at about 1723 cm-1, identifying the presence of almost bare pristine SWCNTs (spectrum B). These two peaks can be used as contrast parameters to visualize the Raman (i.e. chemical) map of the sample. The two opposite visualizations of the same map obtained by using the selected THA and M peaks as contrast parameters are presented in figure 5. In both cases a colour going from blue to red indicates increasing intensity of the peak chosen as contrast parameter. The almost perfect complementarity of the maps is evident, definitely indicating the stable and selective bonding of THA with oxidized carbon nanotubes.
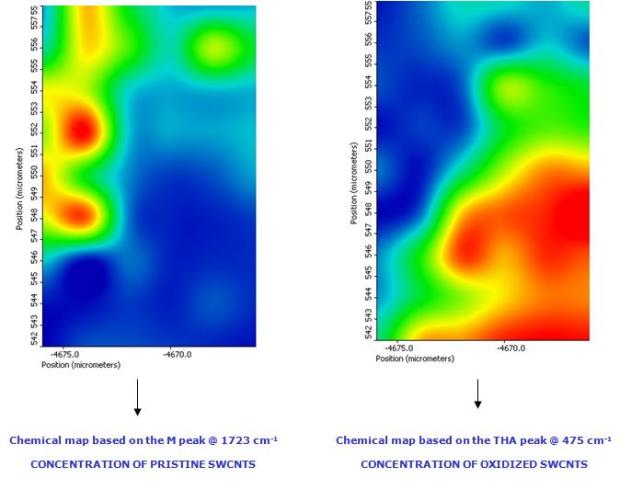
Figure
5. Raman mapping of oxidized carbon nanotubes on an area of 16x10
m2. Figure A and B present, respectively, the visualization of the map
obtained by using the intensity of 475 cm-1 (THA) and 1723 cm-1 (M)
bands as contrast parameters. In both cases a colour going from blue
to red indicates increasing intensity of the peak.
As a first step in drug delivery applications, COOH groups produced by the oxidative treatment can be used to covalently functionalize SWCNTs with PEG molecules to enhance their solubility [3], see figure 6.
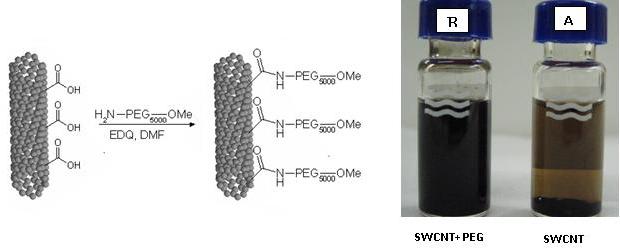
Figure6.
Left: Scheme of SWCNT functionalization with PEG 5 kDa. The reaction
takes place in DMF in presence of EDQ at room temperature for 24 hours.
Rigth: SWCNTs water suspension before (A) and after (B) PEG functionalization.
The suspension B results better dispersed and stable for several weeks
Raman mapping can be also used in in vitro tests to study carbon nanotubes citotoxicity and their interaction and accumulation inside endothelial cells (HUVE cells). Our results 4 demonstrate that SWCNTs are highly biocompatible and not toxic (studies achieved on a specific SWCNT concentration range) and preferentially accumulate on lysosome inside endothelial cells (figure 7).
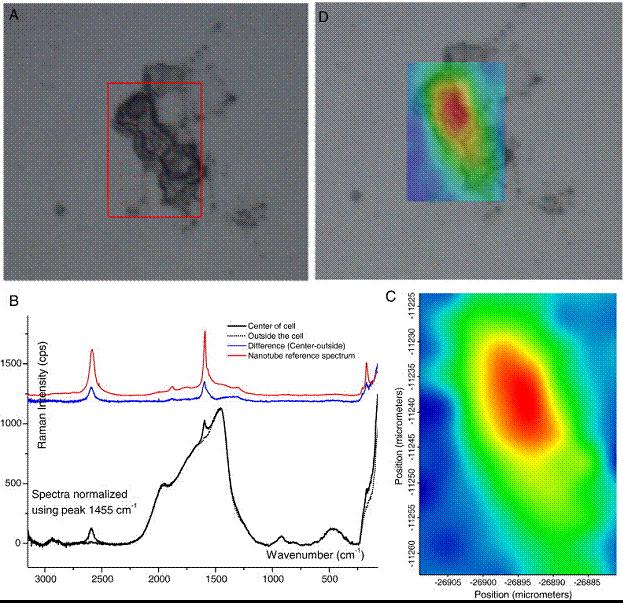
Figure 7. Raman spectroscopy of single HUVE cells in culture. (A) HUVE cell treated with oxidized SWCNTs. (B) The Raman spectra (solid black line) of a point within the SWCNT-treated HUVE cell shown in (A). The dashed line is a spectra from a point outside the cell. The blue line is the difference between the two spectra. The differential curve was essentially superimposable on the spectra obtained from pure oxidized SWCNTs. (C) A "chemical map" generated from Raman spectra from a series of points within the cell shown in (A), with the blue to red gradient indicating increasing G-band intensity. (D) Superposition of the topographic image of the HUVE cell in (A) with the Raman "chemical map", showing the localization of the SWCNTs to the areas confined by the cell.
We then performed in vivo tests on chemically oxidized carbon nanotubes. Preliminary results obtained by Raman spectroscopy investigation of tissue distribution after intravenous injection revealed SWCNT accumulation within the liver and, to a lesser extent, in the spleen (figure 8).
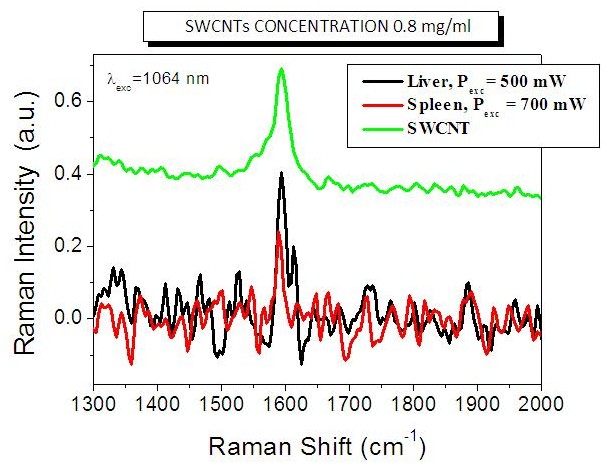
Figure 8. Preliminary results obtained by -Raman spectroscopy investigation of tissue distribution after intravenous injection. The black and red specta were obtained, respectively, on liver and spleen samples at different laser excitation powers. The green spectrum is obtained on the oxidized SWCNTs before injection and is reported as a reference.
"WHO'S WHO" OF THE PROJECT
Valentina Mussi is a staff researcher,
responsible of the carbon nanotubes project. The team is directed by
Professor Ugo Valbusa of the Physics Department
of the University of Genova. Dr. Chiara Biale
is a Ph.D. student of the University of Genova. The project directly
involves Dr. Sonja Visentin of the Department
of Drug Science and Technology (University of Torino), Dr. Nadia
Barbero and Prof. Guido Viscardi
of the Department of General Chemistry and Organic Chemistry and NIS
Centre of Excellence (University of Torino).
Publications related to the project:
[1] S. Visentin, N. Barbero, S. Musso, V. Mussi, C. Biale,, R. Ploeger
and G. Viscardi, "A sensitive and practical fluorimetric test for
CNTs acidic sites determination", Chem. Commun. 46 (2010) 1443-1445.
[2] V. Mussi, C. Biale, S. Visentin, N. Barbero, M. Rocchia, U. Valbusa, "Raman analysis and mapping for the determination of acidic sites on oxidized single walled carbon nanotubes", accepted for publication on Carbon DOI information: 10.1016/j.carbon.2010.05.032
[3] C. Biale, V. Mussi, U. Valbusa, S. Visentin, G. Viscardi, N. Barbero, N. Pedemonte, L. Galietta, "Carbon nanotubes for targeted drug delivery", Nanotecnology, 2009, Proceedings of IEEE-NANO 2009, 9th IEEE Conference, Publication Year: 2009, Pages: 644 - 646.
[4] A.
Albini, V. Mussi, A. Parodi, A. Ventura, E. Principi, S. Tegami, M.
Rocchia, E. Francheschi, I. Sogno, R. Cammarota, G. Finzi, F. Sessa,
D. McClain Noonan, U. Valbusa, "Interactions of single wall carbon
nanotubes with endothelial cells", Nanomedicine: Nanotechnology,
Biology and Medicine, 6 (2010) 277-288.
How to reach us
Last update
6/23/10
